
Got Suds: Taking A Closer Look at Surfactants.
Do I really need to know this?
While you don’t have to be a cosmetic chemist to select a good shampoo, knowing the ingredients that make up your shampoo and how those ingredients work to cleanse the scalp, can be helpful when determining which shampoos will meet your specific hair care needs. Not relying on the claims on the front of the bottle, but rather the knowledge of the ingredients on the back will ensure you are selecting a product that meets your specific needs.
Surfactants
Surfactants can have several purposes. In shampoos, they serve as the active ingredients that do the necessary work of cleaning your scalp. They can range from strong to mild in their cleaning abilities and ultimately determine the overall cleaning strength of your shampoo.
By definition surfactants are detergents designed to help remove oil and buildup from the scalp. They can be naturally derived or synthetic and are key when determining the effectiveness of your shampoo and its ability to meet your wash day needs.
Cleansing the scalp is a necessity, there are several different types of shampoos on the market designed to address the specific needs of your hair. Today, we will take a look at what makes up our shampoos and how these ingredients contribute to the strength of your shampoo and its overall cleansing ability.
“You don’t have to be a cosmetic chemist to select a good shampoo, knowing the ingredients that make up your shampoo can be helpful when determining which shampoos will meet your specific hair care needs.”
The basic make up of shampoo
Shampoos are essentially liquid cleansers made up of detergents and other ingredients that contribute to the look and feel of the shampoo. Most shampoos can contain anywhere from four to twenty ingredients. In most cases the surfactant (active cleansing agent) may only account for a few of those ingredients. Depending on the desired strength of the shampoo and its intended use, shampoos can require a single agent or a combination of surfactants to meet specific cleansing needs.
Secondary ingredients
These ingredients, while not contributing to the cleansing ability of the shampoo, can serve several functions. The most common ingredients in shampoos, other than surfactants, include foaming agents, thickeners, opacifiers (cause shampoo to appear nontransparent vs. clear), sequestering agents (isolate ions to remove water hardness), pH adjusters, preservatives, and miscellaneous ingredients such as perfume.
Ranging in necessity, these agents contribute to the overall aesthetic appeal of the shampoo, but not necessarily its cleansing ability. Some secondary ingredients may have mild surfactant properties giving them added benefit.
How surfactants work
Surfactants are amphiphilic. This means that the cleaning molecule contains both oil attracting (lipophilic) and water attracting (hydrophilic) sites. The oil attracting site attaches to the sebum, while the water attracting site attaches to water. This results in the removal of sebum when the scalp is rinsed with water.
Classification of surfactants
Historically the most common surfactants used were sulfates. Now, in the age of sulfate free cleansers and ingredient conscious consumers, more synthetic and naturally derived alternatives to sulfates are being used.
Anionic, cationic, nonionic, and amphotheric surfactants
Surfactants are classified by the charge (+/-) of their water attracting sites. Anionic surfactants like sodium lauryl sulfate, are negatively charged. Cationic (e.g. benzalkonium chloride) are positively charged. Nonionic (e.g. cetyl alcohol) have no charge, and amphoteric surfactants, like cocamidopropyl betaine, have both a positively and negatively charged water attracting group. For more examples of surfactants, their class, and characteristics see the shampoo surfactant chart below.
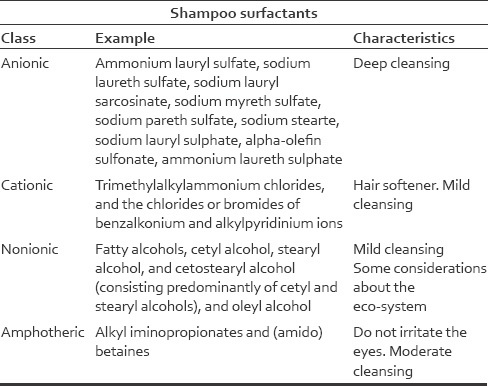
Anionic surfactants
- Derived from fatty alcohols
- Completely removes all sebum from the scalp
- Causes hair to feel harsh, rough, subject to static electricity, and dull
- Their use is unfavorable due to the look and feel of thoroughly cleansed hair
Cationic surfactants
- Limited ability to remove sebum
- Do not produced a significant lather
- Cannot be combined with anionic detergents
- Used in shampoos where minimal cleansing is desired (e.g. daily shampoos designed for permanently dyed or chemically bleached hair)
Nonionic surfactants
- The mildest of all surfactants
- Used in combination with ionic surfactants as a secondary cleanser
- Used to improve the antistatic qualities of a shampoo.
Amphotheric surfactants
- Acts as a cationic surfactant in environments with lower pH
- Acts as an anionic surfactant in presence of higher pH.
- Foams well, leaves the hair in a manageable state, and is found in baby shampoos.
Plant based cleansers
- Mild cleansers
- Natural agents with excellent lathering capabilities
- Combined with synthetic detergents to create their cleansing effects
An ingredient checker like EWG’s cosmetic database or a simple google search can be great resources when trying to get a handle on what purpose the ingredients in your shampoo serve. If you’re looking for an easier approach to quality products, check out Wash day Essentials for examples of quality shampoos to meet your routine and deep cleansing needs.
References




